关键词 > Chemistry30
Chemistry 30 Unit D Module 7 Summative Assessment
发布时间:2024-06-19
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
Chemistry 30 Unit D
Module 7 Summative Assessment
Lesson 1
1. As a chemical system progresses towards dynamic equilibrium, what happens to the rates of the forward and reverse reactions?
2. Compare macroscopic observations to microscopic observations in a system that has established dynamic equilibrium.
3. Identify three conditions that must be met in order for a system to achieve dynamic equilibrium.
|
View the Virtual Investigation “Evidence of a Reversible Reaction” in Module 7 Lesson 1.1. Use the experimental results to answer Questions 4 -11. |
4. When aqueous sodium sulfate is combined with aqueous calcium chloride, describe the empirical evidence that confirms a chemical reaction in the forward direction?
5. Record the data from the Virtual Investigation, “Evidence of a Reversible Reaction” .
Answer (4 Marks)
|
DATA TABLE |
|||||||
|
Trial |
Volume of 0.50 mol/L Na2 SO4 (aq) (mL) |
Volume of 0.50 mol/L CaCl2 (aq) (mL) |
Limiting Reagent |
Excess Reagent |
Mass of filter paper (g) |
Mass of filter paper and CaSO4 (s) precipitate(g) |
Mass of CaSO4 (s) precipitate (g) |
|
1 |
50 |
25 |
|
|
|
|
|
|
2 |
50 |
50 |
|
|
|
|
|
|
3 |
50 |
75 |
|
|
|
|
|
|
4 |
50 |
100 |
|
|
|
|
|
6. Based the data recorded in Question 5, explain why the mass of the CaSO4 (s) precipitate is constant in Trials 2, 3 and 4?
7. Identify which ions should be present in each trial’s filtrate, assuming that this is a quantitative reaction.
Answer (2 Marks)
|
|
|
|
Trial |
Entities expected in filtrate (if reaction is quantitative) |
|
1 |
|
|
2 |
|
|
3 |
|
|
4 |
|
8. Based on the table in Question 7, predict the results when each trial’s filtrate is tested with Ba(NO3 )2 (aq) and Na2 CO3 (aq). Then record the results of the actual precipitate test from the Virtual Investigation.
Answer (8 Marks)
|
|
Trial |
Predicted results when filtrate was tested with Ba(NO3 )2 (aq) |
Predicted results filtrate was tested with Na2 CO3 (aq) |
Actual results when filtrate was tested with Ba(NO3 )2 (aq) |
Actual results when filtrate was tested with Na2 CO3 (aq) |
|
|
1 |
|
|
|
|
||
|
2 |
|
|
|
|
||
|
3 |
|
|
|
|
||
|
4 |
|
|
|
|
||
|
|
||||||
9. Does the empirical evidence recorded in Question 8 support the assumption that the reaction between sodium sulfate and calcium chloride is quantitative? Explain your answer.
10. How does the data from this virtual investigation support the existence of a forward and a reverse reaction? Explain your answer.
11. In theoretical terms, describe how the forward reaction rate and reverse
reaction rate change over time as a chemical system progresses towards
dynamic equilibrium. Be sure to discuss changes to the relative number of collisions between reactants and products. To help you answer this question, refer back to the Virtual Investigation “Evidence of a Reversible Reaction”
(starting at 7:25 min)
Lesson 2
Use the following information to answer the next 5 questions.
|
A technician places 0.50 mol of nitrogen monoxide gas and 0.20 mol of chlorine gas in a 1.00 L sealed container at 100oC. The following equilibrium is established at 4.5 min. 2 NO(g) + Cl2 (g) = 2 NOCl(g) The technician determines that at equilibrium there are 0.30 mol of NOCl, 0.20 mol of nitrogen monoxide and 0.050 mol of chlorine gas. The technician continues recording data for another 3.5 min, noting no change in the concentrations of any of the entities. |
12. Construct a graph that represents the changes in concentrations over time for the three entities involved in the reaction shown in the previous information box.
13. Calculate the percent yield for this reaction (refer to page 680 and 792 of your textbook).
14. Write the equilibrium law expression for this equilibrium.
15. Calculate the equilibrium constant for this reaction.
16. Is the forward or reverse reaction favoured? Support your answer with two pieces of evidence.
17. Write equilibrium law expressions for each of the following equilibrium systems.
a) C6 H6 (l) + Br2 (l) = C6 H5 Br(l) + HBr(l)
b) CH3 COOH(aq) + H2 O(l) = CH3 COO-(aq) + H3 O+(aq)
c) H2 O(g) + Cl2 O(g) = 2 HOCl(g)
d) 4 NH3 (g) + 5 O2 (g) = 4 NO(g) + 6 H2 O(g)
18. Consider the following equilibrium law expression
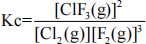
Write the reaction equation that is represented by the above equilibrium law expression.
Use the following information to answer the next question.
|
Consider the following equilibrium system 2 HOCl(g) = H2 O(g) + Cl2 O(g) Kc =110
At equilibrium, a technician determined that the 1.00 L sealed reaction vessel contained 0.18 mol of H2 O(g) and 0.40 mol of Cl2 O(g). |
19. Determine the equilibrium concentration of HOCl(g)
Use the following information to answer the next question.
|
A technician placed 4.0 mol of metaphosphoryl bromide (PO2 Br(g)) in a 2.0 L sealed container. The following equilibrium established 2 PO2 Br(g)= 2 PO2 (g) + Br2 (g) At equilibrium it was determined that the sealed reaction vessel contained 1.8 mol of Br2 (g). |
20. Calculate Kc for the equilibrium shown in the previous information box. Show all work.

