关键词 > LHPhysicalIIIb/0333681
LH Physical IIIb / 03 33681 Chemistry Summer Examinations 2022
发布时间:2024-05-17
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
COLLEGE OF ENGINEERING & PHYSICAL SCIENCES
SCHOOL OF CHEMISTRY
Year 3 Degree of MSci/BSc with Honours
Chemistry
Chemistry with Industrial Experience
Chemistry with Pharmacology
Chemistry with Business Management
Chemistry with a Modern Language
Degree of B.A./BSc Liberal Arts & Sciences
LH Physical IIIb / 03 33681
Summer Examinations 2022
1. (a) Giving full justification, suggest the best technique (or combination of
techniques) and experimental strategy that could be used to investigate each of the following situations (i) to (iii):
[Do not describe all the details of how the methods work from your notes.
Instead you should focus on the key features of the experiment that make it suitable for the situation and explain why other methods maybe less suited.]
(i) the crystal morphology of iridium catalyst particles; (2 marks)
(ii) the absolute configuration of L-methionine in crystalline form; (3 marks)
(iii) the atomic identity and atomic arrangement of an impurity material on a contaminated copper surface. (4 marks)
(b) Explain why the X-ray powder diffraction pattern for fully deuterated L-
glutamic acid (recorded using λ= 1.54 Å) has no significant scattering intensity beyond 2θ = 50。, whereas the neutron powder diffraction pattern recorded for the same material at the same wavelength has peaks of significant intensity well beyond 2θ = 100。. (4 marks)
(c) Explain why the neutron diffraction pattern of KFe(SO4)2 recorded at a
temperature of 5 K contains extra diffraction peaks compared with the neutron diffraction pattern recorded at 15 K, whereas the X-ray diffraction patterns recorded for the same material at 5 K and 15 K are essentially identical. (3 marks)
2. (a) The Fischer-Tropsch process is a chemical reaction between CO and H2 to produce hydrocarbons:
(2n + 1) H2 + n CO → CnH2n+2 + n H2O
The best-known catalyst for the reaction is Ru nanoparticles supported on Al2O3 amorphous particles and the reaction on this catalyst typically takes place at temperatures of 150-300 °C and pressures of one to several tens of atmospheres.
(i) What type of adsorption process needs to occur for each of the reactants for the reaction to take place? Justify your answer with an appropriate energy diagram for the adsorption of hydrogen. (3 marks)
(ii) Which surface characterisation technique would be the most ideal to
characterise the overall morphology of the surface? Justify your answer with an explanation of why this method is suitable. (3 marks)
(iii) Which surface chemistry experimental techniques could be implemented to
determine the electronic effect of the Al2O3 support on the Ru catalyst? (2 marks)
(b) The catalytic activity for the hydrogen evolution reaction changes when
monolayers of Pd are deposited on Au(111) according to the figure below.
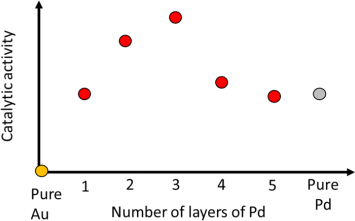
(i) Which surface characterisation technique(s) would be the most ideal to
characterise accurately the surface structure and the interatomic distance of the Pd atoms as a function of the number of layers? Fully justify your answer with an explanation and diagrams of the method(s). (5 marks)
(ii) Which experimental techniques could be implemented to determine the changes in the work function of Pd as a function of the number of layers? Fully justify your answer and explain one of the methods. (3 marks)
3. (a) Adsorption of NO on a Pt(111) surface, at low temperature, results in an ordered
surface structure with a coverage of θ= 0.25. In the EELS spectrum, a peak is observed at 1490 cm– 1, corresponding to the stretching frequency of the NO bond and indicating adsorption in a two-fold site.
(i) Sketch a Pt(111) surface and a NO overlayer structure consistent with this information. Label the unit cell axes clearly on your diagram and give the Wood’s notation to describe this overlayer structure.
(ii) On increasing the coverage of NO, ap(2×1) overlayer structure is formed, for which the peak in the EELS shifts to 1710 cm– 1. Draw the new surface structure, again clearly labelling the unit cell axes and calculate the new surface coverage, θ.
(iii) Draw and label the LEED pattern you would observe for the structure
formed in (ii). For full marks, you must clearly distinguish the reflections arising from the underlying metal surface and those from the adsorbed NO overlayer. (8 marks)
(b) The decomposition of NO over a Pt catalyst to form N2 and O2 follows the rate expression:
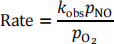
(i) Given that when two species, A and B, compete for adsorption on a surface, the coverage of A is given by the equation
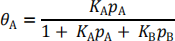
suggest a possible mechanism for the reaction, assuming Langmuir
behaviour and writing out the elementary reaction steps involved. Explain any assumptions you make, particularly explaining why the denominator can be simplified to PO2 . (5 marks)
(ii) With reference to the above rate expression, explain why Pt is not effective for the removal of NO from car exhaust gases. Discuss what is used instead and why it is better than Pt. (3 marks)

