关键词 > MoleculeShapes
Molecule Shapes
发布时间:2024-05-16
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
MoIecuIe Shapes
MODEL 1: Molecule Shapes SimuIation
PART I: ELECTRON GROUPS
1. Explore the Model screen of the simulation. As you explore, answer the following questions.
a. How does adding an atom affect the position of existing atoms orlone pairs?
b. How does adding a Ione pair affect the position of existing atoms and lone pairs?
2. Build the following model in the simulation:  . Check the box“show bond angles”.
. Check the box“show bond angles”.
a. Add additional bonded atoms ( ) to the central atom and write down the bond angles with each addition in the table below until you get to the model with four atoms bonded to the central atom.
) to the central atom and write down the bond angles with each addition in the table below until you get to the model with four atoms bonded to the central atom.
|
Number of additional bonded atoms added to central atom |
Bond angle |
|
0 |
180。 |
|
1 |
|
|
2 |
|
b. Re-build the model in the simulation:  . Repeat part 2a.) but with lone pairs instead.
. Repeat part 2a.) but with lone pairs instead.
|
Number of additional lone pairs added to central atom |
Bond angle |
|
0 |
180。 |
|
1 |
|
|
2 |
|
c. Based on your answers to 2a & 2b above, is the effect of adding bonded atoms and Ione pairs to the central atom similar? Explain why this could be the case.
|
We can think of a bond or alone pair of electrons asan eIectron group. Single bonds, double bonds, and triple bonds each count as one electron group. |
3. How do the electrons in bonds (bonding groups) differ from lone pairs (non-bonding groups)?
4. What happens (in general) to the bond angIe when you add or remove an electron group?
5. Create a model of a molecule with 4 bonding groups around a central atom. Why are the bond angles for a molecule with 4 bonding groups around a central atom not 90。(or right angles)?
6. Click and hold on one end of the molecule and move the atom around. Can you force the
atoms into new configurations (or new arrangements) by pushing atoms around? Why does this make sense based on VSEPR Theory?
7. What does the observation from question 5 above suggest about the configuration of atoms in real molecules?
8. What is the difference between Electron Geometry and Molecule Geometry?
PART 2: DRAWING MOLECULES TO SHOW 3-DIMENSIONALITY
MODEL 2:
Line, Wedge and Dash Drawings
Line: ln the plane of the paper: 
Wedge: Coming forward, in front of the plane of the paper: 
Dash: Going backward, behind the plane of the paper: 
9. Where is each of the 5 atoms in the molecule CHFClBr?
ln the plane of the paper
ln front of the plane of the paper
Behind the plane of the paper
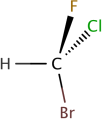
10. Using the Model screen, add bonding groups (●) to the central atom (o). Using lines, wedges and dashes from Model 2, draw each molecule,s shape.
|
Bonding Groups Around CentraI Atom |
Drawing of Shape |
EIectron Geometry |
Bond AngIes |
|
2 |
● 一 o 一 ● |
Linear |
180o |
|
3 |
o |
|
|
|
4 |
o |
|
|
11. ln the following table, identify and draw the moIecuIe geometry.
|
Number of Electron Groups Around Central Atom |
1 Lone Pair |
2 Lone Pairs |
|
3 |
Molecule geometry = |
|
|
4 |
Molecule geometry = |
Molecule geometry = |
PART 3: COMPARING MODEL VS. REAL MOLECULES
12. Explore the Rea/ Mo/ecu/es screen and toggle between different molecules in the drop-down box on the upper-right hand corner of the screen. Note: ignore moIecuIes XeF2 , CIF3, SF4,
XeF4 , BrF5 , PCI5, and SF6 as we are not covering molecules with more than 4 electron groups in this class.
a. Of the molecules H2O, CO2, SO2, BF3, NH3, CH4 , which of these molecules show a difference in bond angIe between“Real”and“Model”. Note: differences in bond angle maybe small.
|
MoIecuIe |
Number of Lone Pairs |
|
|
|
|
|
|
|
|
|
b. What do all of the molecules in the table have in common?
c. What trend do you observe that distinguishes lone pairs from bonding groups? Suggest a reason for this trend.
EXERCISES:
13.A molecule has 2 double bonds on the central atom and no lone pairs. Predict the electron geometry. Predict the molecule geometry. What do you think the bond angles would be?
14. For each of the molecules below, draw the lewis dot structure, determine the electron
geometry, molecule geometry, and bond angles. Draw pictures to show your geometries.
a. CCl4
b. AsF3
c. OF2

