关键词 > R语言代写
Extracorporeal membrane oxygenation
发布时间:2023-10-19
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
Total: 100 points
Write two comprehensive reports (short scientific papers, within 5 pages each) of the following two studies. Please put your code in the appendix.
• Reports exceeding the page limit will be penalized.
• The most relevant output (graphical and numerical) may (and should) be incorporated in the main part of the report, but other outputs together with your R or SAS code should be relegated to the appendix.
• Simply include the outputs from software is not sufficient. You need explain the results, and provide recommendations and conclusions.
• Your report should consist of the following sections:
– The title page with abstract. In the abstract, including the following small sections. Objective, Methods, Results and Conclusion.
– Introduction: Overview of the project.
– Data, analysis and results: state the formulated problems; state the model assumptions; describe what procedures are used to analyze data; carry out model diagnosis if appropriate; present and explain the results.
– Conclusion: state your conclusions, recommendations, suggestions or any other discussions you view important.
– Reference: cite appropriate references.
– Appendix: where you can put additional results or your computer code. Your appendix should be at most 10 pages.
Study 1: (50 points) Extracorporeal membrane oxygenation (ECMO) is an external system for oxygenating the blood based on techniques used in cardiopulmonary bypass technology developed for cardiac surgery. In the literature, there are three welldocumented clinical trials on the evaluation of the clinical effectiveness of ECMO, the Michigan ECMO study (Bartlett, et al. 1985), the Boston ECMO study (Ware, 1989), and the UK ECMO trial (UK Collaborative ECMO Trials Group, 1996).
The UK ECMO trial used randomized allocation with equal proportions, and there were 93 patients in the ECMO and 92 in the conventional treatment for a total of 185. Prior to discharge from hospital, there were 28 deaths in the ECMO treatment and 54 deaths in the conventional treatment. We will use pA = 65/93 and pB = 38/92 as the estimated success probabilities of the ECMO and the conventional treatment, respectively.
We would like to compare treatment A (ECMO) and treatment B (the conventional treatment). Redesign the experiment based on the following information: (i) based on provious information pA = 65/93 and pB = 38/92; (ii) two-side test with level 0.05; and (iii) power
0.9.
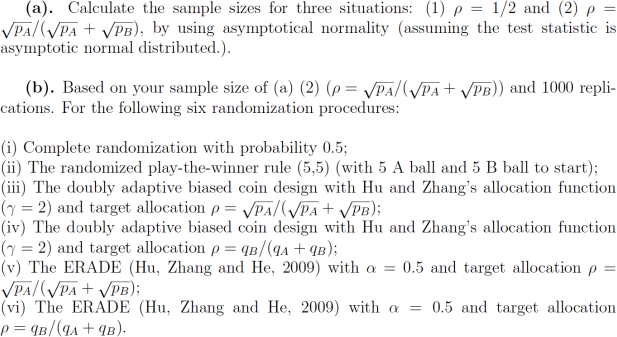
)
We would like to known the number of patients in treatment A, the total failures and the power of tests. Please report their averages and its standard deviations, and the power in Tables. Based on your numerical results, compare the six procedures and discuss their advantages and drawbacks.
(c). Based on your study in (b), recommend one randomization procedure from the six procedures. Please explain why you recommend this procedure.
Study 2: ( (50 points), You may use the ”carat” package for this study) Write a compenhesive report about following A/B testing: We would like to compare treatment A (a new treatment) and treatment B (a placebo). Design the experiment based on the following information: two covariates: medical conditions and smoke status, with 2 and 2 levels respectively, resulting in 4 strata; The covariates’ distribution is replicated in the following Table 1.
Table 1: Distribution of Covariates
two covariates good health condition; smoker 3/20 good health condition; non-smoker 11/20 risk health condition, smoker 5/20 risk health condition, non-smoker 1/20
a). We would like to known the balance properties of these four procedures based on 1000 simulations. Please report your results similar to the Tables 3-6 of the Section 5.3.1 in the lecture notes. Based on your numerical results, compare the four procedures and discuss their advantages and drawbacks for three different sample sizes: n = 30, 60, 120. n patients enter the trial sequentially and their covariates are independently simulated from the multinomial distribution in Table 1. We use the same π = 0.85 and block size
4. The weights are specified in the following way:
- Hu and Hu’s procedure (HH): wo = ws = 1/5 and wm,i = 3/10, i = 1,2.
- Pocock and Simon’s procedure (PS): wo = ws = 0 and wm,i = 1/2, i = 1,2.
For the following four randomization procedures:
(i) Complete randomization with probability 0.5;
(ii) Stratified permuted block randomization with block size 4;(iii) Pocock and Simon’s procedure; (iv) Hu and Hu’s procedure.
b). We consider simulations to study Type I error of hypothesis testing for comparing treatment effects under four designs in a): Pocock and Simon’s marginal procedure, stratified permuted block design, Hu and Hu’s procedure and complete randomization. The following linear model (including two covariates Z1 (medical conditions, Z1 = 1, if good health condition; Z1 = 0, otherwise. ) and Z2 (smoke status, Z2 = 1, if smoke;
Z2 = 0, otherwise. ) ) is assumed for responses Yi,
Yi = µ1Ii + µ2(1 − Ii) + β1Zi,1 + β2Zi,2 + εi,
where εi is distributed as N(0,1), β1 = 2 and β2 = −2. No difference in treatment effects is assumed to study Type I error, i.e., µ1 = µ2 = 0. Consider four working models: The two sample t-test; lm(Z1); lm(Z2)) and lm(Z1,Z2) and two different sample sizes: n = 30, 60. Report your result as Table 1 of Ma, Hu and Zhang (2015, JASA) and discuss your founds. (Note: BS − t is not required here.).
c). We compare power for different hypothesis testing methods under four designs in
a): Pocock and Simon’s marginal procedure, stratified permuted block design, Hu and Hu’s procedure and complete randomization.The same model as in b) is used, except that difference exists between treatment effects µ1 and µ2, i.e., µ1 − µ2 6= 0. Consider four working models: The two sample t-test; lm(Z1); lm(Z2)) and lm(Z1,Z2) and two different sample sizes: n = 30, 60. Report your result similar to Table 2 of Ma, Hu and Zhang (2015, JASA) for µ1 − µ2 = 0,0.1,0.2,0.3,0.4,0.5,0.6,0.7,0.8,0.9,1 and discuss your founds. (Note: BS − t is not required here. Also variance adjustments are not required for the four models. ) (Hint: You should read the paper of Ma, Hu and Zhang (2015, JASA) for details of a similar study.).
REFERENCES
[1] Hu, F. and Rosenberger, W. F. (2003). Evaluationg response-adaptive randomization procedures for treatment comparisons. Journal of the American Statistical Association 98 671–678.
[2] Hu, F. and Rosenberger, W. F. (2006). The Theory of Response-Adaptive Randomization in Clinical Trials, John Wiley and Sons, Inc., New York.
[3] Hu, F. and Zhang, L.-X. (2004). Asymptotic properties of doubly adaptive biased coin designs for multitreatment clinical trials. The Annals of Statistics, 32 268–301.
[4] Hu, F., Zhang, L.-X. and He, X. (2009). Efficient randomized adaptive designs, Annals of Statistics. 37, 2543-2560.
[5 ] Hu, Y. and Hu, F. (2012). Asymptotic Properties of Covariate-Adaptive Randomization. Annals of Statistics. 40, 1794-1815.
[6 ] Ma, W., Hu, F. and Zhang, L.X. (2015). Testing Hypotheses of Covariate-Adaptive Randomized Clinical Trials. Journal of the American Statistical Association. 110, 669-680.
[7 ] Ma, W., Qin, Y., Li, Y. and Hu, F. (2020). Statistical Inference for Covariate-Adjusted Randomization Procedures. Journal of the American Statistical Association, 115, 1488-1497.
[8] UK Collaborative ECMO Trial Group. (1996), UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet, 348 75–82.

