ENGN8833 Industrial Energy Efficiency and Decarbonisation HOMEWORK 3 – Chemical Processes
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
ENGN8833
Industrial Energy Efficiency and Decarbonisation
HOMEWORK 3 – Chemical Processes
Due date: 5 pm, 19 Apr 2024
This homework involves analysis of the syngas production part of a methanol plant, and is an extract from Chapter 13 (by RW Rousseau and B Keyes) of the book RM Felder and RW Rousseau, 2000, Elementary Principles of Chemical Processes, 3rd Ed, Wiley. The aim of this homework is for you to actively learn the process of applying basic thermodynamic and chemistry principles to a large-scale industrial process. It contributes primarily to learning objective 2 of this course (thermodynamic analyses of industrial processes), but will also facilitate your later work in objectives 4 and 5, where we look at ways to decarbonise processes like this one. Syngas production has been selected for this homework because of its potential future relevance to polymer and synthetic fuel production from biomass.
Introduction
Incompletely labelled flowcharts for the overall process and a simplified version of the reformer, are given in Figs 1 and 2. The following is a process description that includes additional details.
Steam methane reformer
A mixture of carbon monoxide, hydrogen, and carbon dioxide is produced by steam reforming, a process in which natural gas and steam are mixed and reacted in a reformer operated at 1.5 MPa. Natural gas may be assumed to consist entirely of methane (CH₄), although other compounds may be present in small concentrations. In the present process, steam and natural gas are fed to the reformer in a ratio of 3.0 moles of steam per mole of methane. The reformer consists of an arrangement of vertical tubes filled with nickel-impregnated ceramic catalyst. Rows of these tubes are located inside an insulated firebox, where they are heated by the combustion of natural gas.
The natural gas and steam that are blended to become the reformer feed enter the process at 30°C and 210°C, respectively. The mixture is preheated to 450°C by exhaust gas from the firebox, and it is introduced to the reformer through a header that distributes the mixture evenly among the parallel reformer tubes. Two key reactions occur: the steam-reforming reaction itself,
CH4 + H2O(g) = CO + 3 H2 (1)
and the water–gas shift reaction,
CO + H2O = CO2 + H2 (2)
The product gas leaves the reformer at 832°C and 1.5 MPa.
Energy efficiency in steam reforming is improved by recovering heat from the burner exhaust gas, which leaves the firebox at 960°C. The exhaust gas is cooled in a series of heat-exchange operations that preheat the reformer feed streams to 450°C, produce superheated steam at 4.5 MPa and 100°C superheat from boiler feedwater at 30°C, and preheat the combustion air to 300°C. The superheated steam is used to drive turbines elsewhere in the process or it can be exported, for example to generate electricity. The burner exhaust gas leaves the heat-recovery units and enters a stack at 150°C for release to the atmosphere.
Problems
The context of this case study is the possible purchase of a plant using the above described methanol production technology. A company is offering to sell a complete plant that produces 420,000 t/y of specification-grade methanol, when operating 350 d/y. The ultimate aim of this case study is to evaluate what price should be offered to that company for building the plant.
1. The process objective can be described most simply as converting methane and water into methanol and hydrogen, and then purifying the methanol so that it meets specifications. The overall process stoichiometry is given by the following relationship:
CH4 + H2O → CH3OH + H2 (3)
From this statement, estimate the feed rates of the natural gas (both in kmol/h and in m³/h at STP) and steam (kmol/h, kg/h) fed as reactants (as opposed to fuel) to the reformer. (Note: The requested estimate neglects formation of by-products and the carbon monoxide and carbon dioxide lost in the purge stream.) (5 marks)
2. Seven percent excess air is used in burning the reformer fuel; it is drawn into the system at 25°C and 60% relative humidity. Estimate the average molecular weight of the air.
(Compare the value with that obtained for dry air. Why does the result differ from this value?) Determine the flow rate of this stream (kmol, m³) per kmolof natural gas burned. (5 marks)
Hint: The relative humidity (φ) of air is defined as the ratio of the partial pressure of water vapour to the saturation vapour pressure at a given temperature as follows:
φ = p sat(p H)2(T(O)) (4)
The molecular weight of moist air is calculated as:
Mmoist air =( 1 − y H2 O)(0.21 × MO2 +0.79 × MN2)+yH2 O MH2 O (5)
where y H2 O is he mole fraction of water vapour and M is the molecular weight.
3. What are the compositions (mole and mass fractions) and volumetric flow rates (m³/kmol
CH4 fed to burners) of (a) the effluent gas from the reformer burners and (b) the gas entering the stack? What is the specific gravity, relative to ambient air (25°C, 1 atm, 60% relative humidity), of the stack gas as it enters the stack? Why is this quantity of importance in designing the stack? Why might there be a lower limit on the temperature to which the gas can be cooled prior to introducing it to the stack? (10 marks)
Use a methane feed rate to the reformer of 1600 kmol/h as a basis for subsequent calculations.
4. The primary purpose of the reformer is to convert methane and water to carbon monoxide
and hydrogen (Eq. 1). The extent of this reaction is limited by chemical equilibrium.
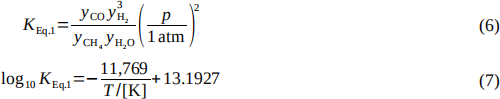
where yi are the mole fractions of the mole fractions of the corresponding species, p is the pressure and T is the temperature in kelvin.
(a) If Eq. 1 were the only reaction occurring in the reformer, estimate the composition of the product gas that would be leaving the reformer and the conversion of CH4, assuming the product stream has achieved chemical equilibrium at 832°C and 1.5 MPa. What would be the total flow rate of this stream (kmol/h, kg/h)? (10 marks)
(b) It is specified that the molar ratio of steam to methane fed to the reformer is 3.0, whereas the stoichiometric ratio for the reforming reaction (Eq. 1) is 1 mole of water per mole of methane. Estimate the conversion of methane for steam-to-methane feed ratios of 1:1
and 2:1, and compare these to the conversion in part (a). Based on your results, explain in your own words why you think the ratio of 3 moles of steam per mole of methane was chosen for the process. (5 marks)
5. As pointed out in the Process Description, the water–gas shift reaction (Eq. 2) occurs in the reformer along with the reforming reaction (Eq. 1). It is controlled by chemical equilibrium,
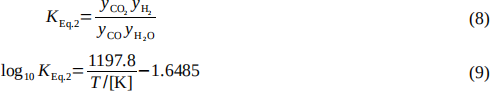
with similar nomenclature to the previous question.
(a) Taking into account the occurrence of reactions given by both Eq. 1 and 2, estimate the composition of the product gas leaving the reformer and the conversion of CH4, assuming the product stream leaving the reformer has achieved chemical equilibrium at 832°C and 1.5 MPa. What is the total flow rate of this stream in both kmol/h and kg/h? What effect does the water–gas shift reaction have on the production of CO at the reformer conditions? (10 marks)
(b) The ratio of CO to H2 can be an important variable in efficient use of raw materials. In this case study a 3:1 steam-to-methane molar ratio of feed streams was specified.
Determine how this feed ratio affects the ratio of CO to H2 in the product from the reformer assuming the reaction products are in chemical equilibrium at 832°C and 1.5 MPa. (7 marks)
6. Quantitatively demonstrate that high temperatures and low pressures favour the formation of CO and H2 in the reformer. Do this by calculating and then plotting the production rates (kmol/kmolof CH4 fed) of CO and H2 in the reformer product stream over the temperature range 750–950°C at 1.2, 1.6, and 2.0 MPa. Furthermore, construct plots showing the effect of temperature and pressure on selectivity (defined as kmol CO formed per kmol CO2 formed) over the same range of conditions. Assuming that your results support the hypothesis that high temperatures and low pressures favour the formation of CO and H2, speculate as to why the temperature and pressure are at the values specified in the Process Description (832°C and 1.5 MPa) rather than at a higher temperature and lower pressure.
(15 marks)
7. The reformer product gas leaves the reformer at 832°C.
(a) Using the flow rate of the product gas determined in Question 5, calculate the rate (kJ/h) at which heat must be transferred from the combustion gases to the gases flowing through the inside of the reformer tubes. (5 marks)
(b) What is the required flow rate of natural gas (kmol/h and m²/min at STP) to the reformer burners? Assume that the natural gas is burned to completion in the reformer firebox and that the combustion gases leave the firebox at 960°C. (5 marks)
(c) The thermal efficiency of the firebox may be defined as the percentage of the lower heating value of the fuel transferred to the reformer gases. Estimate the lower heating value of methane and, assuming the combustion gases leave the firebox at 960°C, the corresponding thermal efficiency of the firebox. (5 marks)
8. The heated tube length in the reformer is 10 m and the external diameter of the tubes is
10.5 cm. If the rate of heat transfer (Q) from the combustion gases in the firebox to the reformergases were accomplished entirely by convection, the following equation would apply:

where Uo is an overall heat transfer coefficient based on the external surface area of the
reformer tubes in the firebox, Ao is the total external surface area of the tubes, and Δ T![]() LM is an average difference between temperatures of the heat source (combustion gases) and the heat sink (reformer reaction gases):
LM is an average difference between temperatures of the heat source (combustion gases) and the heat sink (reformer reaction gases):

where Δ T 1 and Δ T2 are differences in temperature between the reformer gas and combustion gas at the inlet and at the outlet of the firebox. If the combustion gases are assumed to have a constant temperature in the firebox of 960°C (i.e., they are perfectly mixed), and Uo ≈300 W/m²K, what is the required number of tubes in the firebox? In fact, a large fraction of the heat transferred to the tubes is accomplished by a mechanism other than convection. What is that mechanism? What will consideration of this additional mechanism mean in terms of the number of tubes required in the firebox? (10 marks)
9. Recently, a project in Australia has been proposed for ‘solar methanol’ production
(https://is.gd/nl50tC). Drawing on your analysis above, briefly describe how this process works, and its significance as an effort to decarbonise global industry. (8 marks)
Total: 100 marks
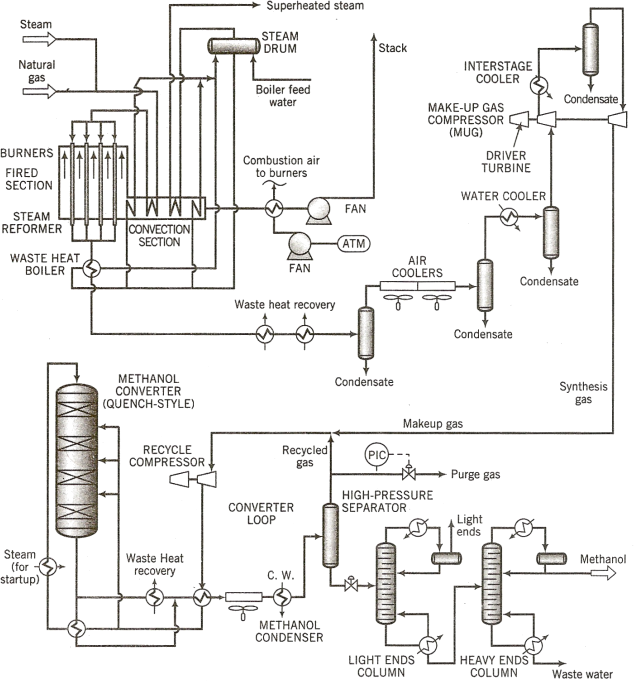
Figure 1. Overall methanol synthesis process
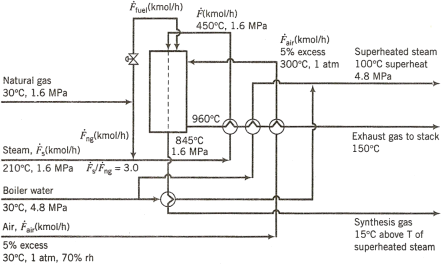
Figure 2. Steam methane reformer
2024-04-13