CBE 9250b “Advanced Biomaterials Engineering” Final Examination – April 2023
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
CBE 9250b “Advanced Biomaterials Engineering”
Final Examination – April 2023
Open Book Exam
Question #1 (25 Points)
(a) The presence of a metallic orthopaedic implant results in stress shielding which is generally thought to be deleterious. The level of compressive stress in an implant containing bone, sb is affected by the bone cross-sectional area fraction, ab and the stiffness of the bone and implant, Eb and Ei
(i) Assuming that the bone and implant are loaded in uniaxial compression and the compression
normal strain is the same in both the bone and implant, show that
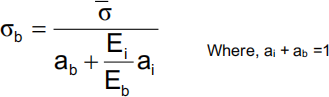
Where, s is the compressive stress averaged over the area of the bone and implant
(ii) If the stiffness of the metallic implant is orders of magnitude larger than the stiffness of the bone and the area fractions of the bone and the implant are approximately equal, show that the result in part (a) reduces to
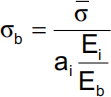
How do changes in ai and Ei/Eb affect the compressive stress applied to bone? 6 points
(b) Comment on stress shielding and cell biomaterial interaction of trabecular tantalum metal reported in the following references listed below and what be your suggestions to minimize this problem? 6 points
Bencharit, S., Byrd, W. C., Altarawneh, S., Hosseini, B., Leong, A., Reside, G., Morelli, T. and Offenbacher, S., “ Development and Applications of Porous Tantalum Trabecular Metal-Enhanced Titanium Dental Implants” Clin. Implant Dent. Relat Res., 2014, 16(6): 817-826.
Keotsostathis, S.D., Tsakotos, G. A., Papakostas I., Mcheras, G. A. “Biological Processes at Bone – Porous Tantalum Interface. A Review.” J. Orthopedics, 6(4) e3 (2009).
Sevilla, P., Aparicio, C., Planell, J.A., Gil, F.J., Comparison of the Mechanical Properties between Tantalum and Nickel-Titanium Foams Implant Materials for Bone Ingrowths Applications, J. of Alloys and Compounds, 439, 67-73 (2007).
Balla, V. K., Bodhak, S., Bose, S., Bandyopadhyay, A. “Porous Tantalum Structures for Bone Implants: Fabrication, Mechanical and In Vitro Biological Properties” Acta Biomaterialia, 6(8) 3349-3359 (2010).
(c) Cortical bone consists of 69% hydroxyapatite and 31% collagen. The elastic modulus of cortical bone in GPa is (Et) and the elastic moduli of hydroxyapatite and collagen are (Em) and (Ec) respectively. If the volume fraction of the hydroxyapatite is (Vm), construct a plot of Et versus Vm using the Voight and Reuss models assuming Em/Ec = 100. At Vm = 69%, what are the modulus values according to the Voight and Reuss model? Compare these values with the actual elastic modulus of cortical bone (17 GPa). Explain why the actual value is different from the ones calculated from the Voight and Reuss models. 7 points
(d) Comment on the behaviour of metallic implants cemented with bioactive bone cements compared to cementless or implants cemented with normal PMMA bone materials. Justify your answers with recent references from the literature. Provide a list of at least two recent references you used to justify your answer. 6 points
Question #2 (25 Points)
(a) One way of strengthening hydroxyapatite (HA) and ZrO2 to be used for orthopedic implants is to coat them onto Co-Cr and Ti-6Al-4V alloys to act as supports against tensile failure. Thecoating process is usually conducted at high temperature and then cooled down to room temperature. Assume that the critical temperature (ΔTcr = 800ºC) and the strain in the ceramic is equal to the strain in the alloy. The elastic modulus and coefficient of thermal expansion for each of the bioceramics and metal alloys are listed below: 10 points
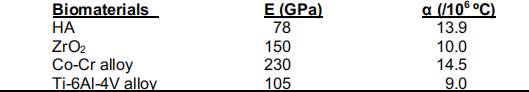
bioceramic-metal alloy interface? Calculate the magnitude of those stresses and explain which interface(s) is/are expected to fail.
ii) Calculate the compressive strength of each of the above two bioceramics when they are not bonded to the above two alloys.
iii) If the stiffness of TZP is significantly lower than that of alumina, describe the toughening mechanism that makes the fracture toughness of TZP significantly higher than that of alumina.
(b) A bioengineer is asked to make a composite material from Kevlar® fiber and poly (methyl methacrylate) PMMA resin (as a matrix) for a bone plate. The following data are given:
Material E (GPa) Density (g/cm3) Strength (MPa)
Kevlar® fiber 130 1.45 2700
PMMA 3 1.20 70
(i) What is the amount of Kevlar® fiber in weight and volume percent required to make the modulus of the plate 100GPa? 1 point
(ii) How many fibers are required if the diameter of the fiber is 5 µm and the cross-sectional area of the specimen is 5 cm2 1 point
(iii) How much stress in the fiber direction can the composite take? 1 point
(iv) Calculate the modulus of elasticity of the composite plate in the fiber direction. 2 points
(v) Calculate the modulus of elasticity of the composite plate in the direction perpendicular to the fiber direction. 2 points
(vi) Compare the above two calculated values and estimate a modulus value if the composite plate is isotropic. 2 points
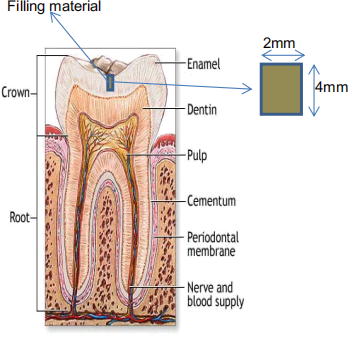
(c) Dental filling materials can readily experience a temperature differential on the order of 50°C in normal daily function. (Consider the consumption of a cold or hot beverage). Also consider a dental filling material that is cylindrical in shape as shown in the figure below. If this dental filling is 2 mm in diameter and 4 mm in length, what is the expected volumetric expansion for a filling material made of alumina with a thermal expansion αalumina = 8.5 x 10-6 /°C? How does this compare to dental fillings made with resin, αresin = 85 x 10-6 /°C and gold alloy, αgold alloy = 14 x 10-6 /°C? Assume a temperature change of 50 °C and the dental filling is assumed to deform isotropically. The thermal expansion coefficient for enamel is 8 x 10-6 /°C. The elastic modulus for alumina is 400 GPa, for the resin is 2.5 GPa and for the gold alloy is 95 GPa. Calculate the one-dimensional forces owing to these thermal expansions. Comment on the result of your calculations. 6 points
Question # 3 (25 Points)
Due to increasing incidence of peripheral arterial occlusive diseases and coronary heart diseases, there is a tremendous need for small diameter vascular grafts. To overcome the limitations of synthetic grafts and autologous grafts, two composite vascular grafts were developed (see the following table).
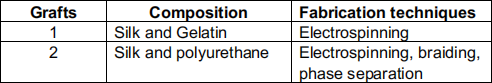
Circumferencial tests were performed on both grafts and porcine aorta till samples break, the following data was collected and displayed in the chart below.
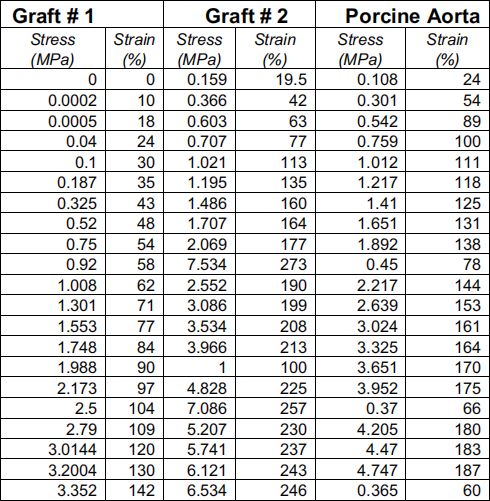
1. Plot the stress-strain curve for each specimen into one graph, determine the ultimate tensile stress strain at break for grafts and porcine aorta.
2. Natural blood vessels have a nonlinear mechanical behavior where they exhibit a two-stage
behavior- a toe region followed by a linear region.
a. Identify and label the two regions for all samples.
b. Determine the elastic modulus for all samples.
3. Consider the structure and composition of blood vessels.
a. Explain why aorta exhibits a two-stage behavior under an increased strain.
b. If the longitudinal or transverse tensile test is performed on the aorta, would you expect a similar mechanical behavior? What would be the change in elastic modulus?
c. Justify the material selection and material combination (i.e., silk with gelatin and silk with polyurethane) for grafts # 1 and # 2. Justify the choice of your model.
4. Several models have been proposed in the literature to fit the mechanical response of soft tissues, using an appropriate model to fit the data for grafts # 1 & # 2. Justify the choice of your model.
5. Use the best fit model, determine the elastic modulus for graft # 1 # 2. Compare your results with the results from part 2b, which value(s) you think is more credible?
6. Briefly outline experimental procedure for the circumferential tensile tests for vascular conduits.
7. Ro complete the mechanical profile of the two grafts and evaluate the feasibility as vascular replacements, list any other mechanical properties you would like to study and
a. Explain why these properties are crucial for vascular implant.
b. Outline the testing that could be performed to evaluate the mechanical properties you selected.
8. Compare the three graphs generated in part 1, which graft will you choose as an arterial graft? Considering the structure and properties differences between arterial and venous vessels, which synthetic graft would be more suitable for venous replacement? Use data/references from the literature to justify your choice.
9. If the intended use of graft # 1 is an arterial replacement, suggest possible improvements in composition and fabrication techniques to closely mimic mechanical behaviors of native arterial vessels.
Question # 4 (25 Points)
a. There is a new drug-eluting stent on the market. This stent technology uses diffusion- controlled release from a biostable polymer matrix to deliver an anti-inflammatory agent in the polymer matrix and the diffusion coefficients for the three formulations are 9.5 x 10- 11 cm2/s (slow), 7.0 x 10- 10 cm2/s (medium) and 5.2 x 10-9 cm2/s (fast).
i. Assume that the complex geometry of a stent can be approximated as a planar slab with diffusion in one dimension and the coating thickness is 1 mm. Plot the release profiles on one graph. Determine the half-life of each formulation.
ii. A patient who has an arterial lesion that will require drug treatment for a duration of no less than 30 days, but complete by 2 months. Which formulation would you recommend?
b. A mixture of two polysaccharides (A and B) was prepared as polymer matrix to provide controlled release of therapeutic molecules. Nimesulide was selected as the model drug and suspended in the mixture. The resultant gel was dried and pressed into tablets.
Two tablet formulations were tested in the study. In the first formulation, A was cross-linked with B using sodium trimetaphosphate. In the second formulation, no cross-linker was used.
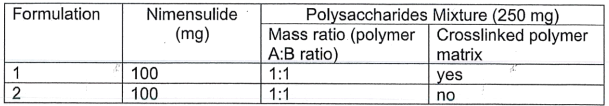
Liquid uptake capacity is important to a drug delivery system. The swelling degree of the tablets was studied by immersing the system in media at two pH values (pH 2.0 and pH 7.4). The results were presented below.
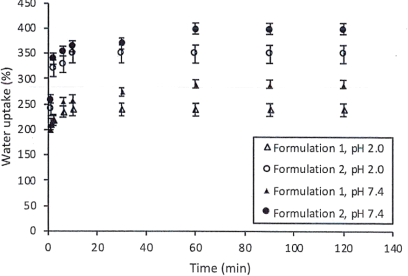
1. Comment on
a. Effect of pH on the liquid uptake.
b. Effect of degree of crosslinking on the liquid uptake.
Drug release study of the tablets was performed in media with sequential pH values to simulate segmental pH environments in the digestive system. More specifically, tablets were soaked in acidic media (pH2.0) for 120 min. Then the pH was neutralized and maintained at 7.4 (from t=120 min. to t=360 min). Surfactant was added to all drug release media to promote the solubility of Nimensulide in the media.
Nimensulide release data was retrieved and displayed in the following table.
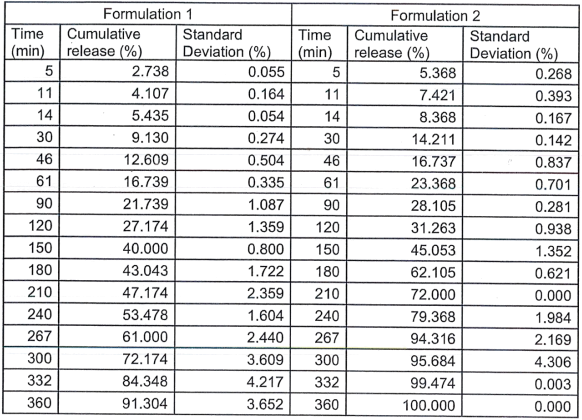
2. Plot the two drug release profiles in the same graph, indicate the pH(s) at which the experiments were performed.
3. Relate the swelling profile to drug release profile, what is happening to the polymer matrix during drug release? How does the swelling of the matrix affect drug release?
4. There are several mathematical models can be used to interpret the drug release mechanism. Using the data provided and
a. Determine the best model to fit the data, state the assumptions and calculate the model parameters.
b. Describe the possible releasing mechanism.
5. If the delivery system was transferred to another release media (pH=5.0) at t=240 min, how would the release curve look like? Plot the release profile, suggest, and explain in detail, an appropriate application for this delivery system. How could you alter this system to better serve the purpose?
2024-04-05