Building physics Exercise class 1
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
Building physics
Exercise class 1
FIRST LAW IN CLOSED SYSTEMS
1.1 Specific heat calculation
In order to calculate the specific heat of iron, a 1 kg body of this material (PFe = 7874 kg/m3) is immersed in a 2 L water bath calorimeter (adiabatically isolated, PH2O = 1000 kg/m3, cH2O = 4.186 kJ/(kg K)). Knowing that the initial temperature of water is TH2O = 20 °C, the initial temperature of iron is equal to TFe = 100 °C and that the equilibrium temperature is 24.04 °C, calculate the specific heat of iron.
[CFe = 0.444 kg K/KJ
1.2 Equilibrium temperature
A empty teapot (specific heat 840 J/(kg K), mass 0.3 kg) with a volume of 0.5 latTi= 20 °C is filled with hot water at 100 °C (PH2O = 1000 kg/m3 and specific heat of 4186 J/(kg K)) to prepare tea. Calculate the equilibrium temperature reached after a certain amount of time, without considering the heat exchanged with the environment.
Then, it is requested to prepare, on the same teapot, half a liter of herbal tea at 50 °C, with hot water at 100 °C and cold water at 15 °C. How much cold water do you need?
[Teq = 91.4 °c; MH2o cold = 0.273 kg]
1.3 First principle of thermodynamics
Consider a device like the one used by Joule for his experiments. The system is composed of a well- insulated vessel (adiabatic) that contains 1 L of oil (ρoil = 850 kg/m3, coil = 1.85 kJ/(kg K)).
Inside the vessel there is also a water wheel connected to a 100 kg body. This body falls due to gravity (g = 9.81 m/s2) and work is transferred to the system with oil by means of pulleys and crank mechanisms.
Knowing that this mass makes a vertical uniform rectilinear motion of 1 m (initial and final velocities are the same), calculate the temperature variation of the fluid inside the vessel.
(Neglect friction due to air and to the pulley and crank system).
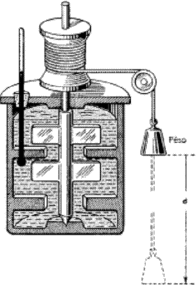
[∆Toil = 0.624 K]
PROPERTIES OF MATTER
1.4 Pressure of a saturated liquid inside a vessel
A solid vessel contains 50 kg of water in saturated liquid conditions, at a temperature of 90 °C. Please calculate the pressure inside the vessel and its volume.
[p = 70. 183 Kpa; V = 0.0518 m3]
1.5 Volume variations and exchanged heat during evaporation
A 200 g mass of water, initially in saturated liquid conditions, is completely evaporated under a constant pressure of 100 kPa. Please calculate the variation in volume and the amount of heat provided to the water.
[∆V = 0.3386 m3 ; Q = 451.5 KJ]
1.6 System conditions
A pressure vessel with a volume of 3 dm3 contains 2 g of water at a pressure of 70 kPa. Please calculate the state of the water inside the vessel (subcooled/saturated liquid, two-phase mixture, saturated/superheated vapor). In case of two-phase mixture, please calculate the mixture quality.
Then, calculate the internal energy and enthalpy of the system.
[Two − pℎasemixtuTe, x = 0.619; U = 3.373 KJ; H = 3.578 KJ]
1.7 Isobaric heating
10 liters of water at T=20 °C and P=1 atm are contained in a thermally isolated vessel with a piston free to scroll. Then, a 1kW resistance is switched on and heats up water. How long does the resistance needs to be turned on to achieve a two-phase mixture with a quality of 0.8?
[∆t = 21360 s]
1.8 Superheated vapor
Water vapor at T= 400 °C and P= 30 bar is contained in a 3 m3 solid vessel. The vessel is cooled until saturated vapor conditions are obtained. Please determine the mass in the system, temperature and pressure at the end of the cooling phase. To conclude, calculate the heat transfer.
[M = 30. 19 Kg; T2 = 212°C; p2 = 2011Kpa; Q = −10098 J]
2024-01-15