ME 104 Thermodynamics [F23] Homework Assignment 9
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
ME 104 Thermodynamics [F23]
Homework Assignment 9
[Due Mon 10/30]
Text: Thermodynamics: An Engineering Approach, Y. Çengel and M. Boles, McGraw-Hill, 10th Edition, 2024. The link to the book is provided on the course site.
Problem 1: [7–86] Air at 12oC is contained in a piston-cylinder device. Air is now allowed to expand in an irreversible process until the air temperature is 450 K and the air volume triples. Accounting for the variation of specific heats with temperature, determine the entropy change of the air.
Problem 2: [7–89] A piston–cylinder device contains 0.75 kg of nitrogen gas at 140 kPa and 37°C. The gas is now compressed slowly in a polytropic process during which PV^1.3 =constant. The process ends when the volume is reduced by one-half. Determine the entropy change of nitrogen during this process using (a) constant specific heats at room temperature and (b) variable specific heats.
Problem 3: [7–94] Air at 27°C and 100 kPa is contained in a piston–cylinder device. When the air is compressed adiabatically, a minimum work input of 1000 kJ will increase the pressure to 600 kPa. Determine the mass of air in the device (a) using constant specific heats at 300 K and (b) accounting for the variation of specific heats with temperature.
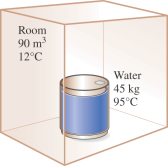
Problem 4: [7–100] A container filled with 45 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer between the water and the air in the room. Using constant specific heats, determine (a) the final equilibrium temperature, (b) the amount of heat transfer between the water and the air in the room, and (c) the entropy generation. Assume the room is well sealed and heavily insulated.
2024-01-08