ME 104 Thermodynamics [F23] Homework Assignment 8
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
ME 104 Thermodynamics [F23]
Homework Assignment 8
[Due Wed 10/25]
Text: Thermodynamics: An Engineering Approach, Y. Çengel and M. Boles, McGraw-Hill, 10th Edition, 2024. The link to the book is provided on the course site.
Problem 1: [7–39] A rigid tank is divided into two equal parts by a partition. One part of the tank contains 2.5 kg of compressed liquid water at 400 kPa and 60°C while the other part is evacuated. The partition is now removed, and the water expands to fill the entire tank. Determine the entropy change of water during this process, if the final pressure in the tank is 40 kPa.

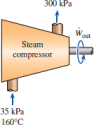
Problem 2: [7–52] Water vapor enters a compressor at 35 kPa and 160°C and leaves at 300 kPa with the same specific entropy as at the inlet. What are the temperature and the specific enthalpy of water at the compressor exit?
Problem 3: [7–58] A piston–cylinder device contains 5 kg of steam at 100°C with a quality of 50 percent. This steam undergoes two processes as follows:
1-2 Heat is transferred to the steam in a reversible manner while the temperature is held constant until the steam exists as a saturated vapor.
2-3 The steam expands in an adiabatic, reversible process until the pressure is 15 kPa.
(a) Sketch these processes with respect to the saturation lines on a single T-s diagram.
(b) Determine the heat transferred to the steam in process 1-2, in kJ.
(c) Determine the work done by the steam in process 2-3, in kJ.
2024-01-06