03 FASCINATING PHENOMENON, POSTLAB QUESTIONS
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
03 FASCINATING PHENOMENON, POSTLAB QUESTIONS
ACID-BASE REACTION
1. Describe the recommended technique in the lab manual for mixing the solutions in the test tube. 2. Explain in one sentence why mixing is important.
3. When bromothymol blue (BTB) is used to monitor the pH of fish tanks, we try to get a green color. If a fish owner tested the water and got a very yellow solution, should he add some acid or some base to adjust the pH properly? Explain using the Claim, Evidence, and Reasoning format.
4. Adding a drop of hydrochloric acid to the solution of bromothymol blue changes the pH from neutral to acidic. There is so very little bromothymol blue in the solution that the pH change is about this same as if the hydrochloric acid were dropped into pure water. That is why these
questions ask about adding HCl to pure water.
a. What is the concentration of hydrochloric acid when a drop of 0.10 M HCl is added to
2.0 mL of pure water? Assume 20 drops to 1.0 milliliter. For help with dilution calculations: https://www.youtube.com/watch?v=QYK3Aj-IUIs (please read the comment section
where Professor Dave corrects his “stupid mistake”).
b. The hydrogen ion concentration in pure water is 10-7 M. How many times greater did the hydrogen ion concentration become when the drop of HCl was added to 2 mL of pure water?
c. Using the relationship that pH=-log[H+] (and for the HCl solution that pH=-log[HCl]) what is the pH change of the pure water when a drop of acid was added to the water? For help
with the calculation of pH. https://www.khanacademy.org/science/ap-chemistry-
beta/x2eef969c74e0d802:acids-and-bases/x2eef969c74e0d802:introduction-to-acids-and-
bases/v/the-ph-scale Use this information do describe why it was difficult to get the
solution to return to a green color. Bromothymol blue is some shade of green between pH 6.5 and 7.5.
5. For the reaction of hydrochloric acid and sodium hydroxide,
a. Write a balanced equation.
b. Write the net ionic equation.
6. Bromothymol blue is an acid, but it is sold as the sodium salt of that acid and has the formula
C27H27Br2NaO5S (referred to as NaBTB). The solution provided to you in the lab was made by weighing 0.010 g of NaBTB, placing it in a flask, and diluting the solution up to exactly 100 mL. This solution is described as having a concentration of 0.010% (w/v). This designation means the numerator (the letter w in the expression w /v) is in units of weight (grams) and the denominator (the letter v in the expression w/v) is in units of volume (milliliters). What is the molarity of the solution?
7. Write the reaction of the base version of bromothymol blue with HCl. Use the abbreviation NaBTB not the actual chemical structure of bromothymol blue.
Figure 1: Example of a neat and legible table.
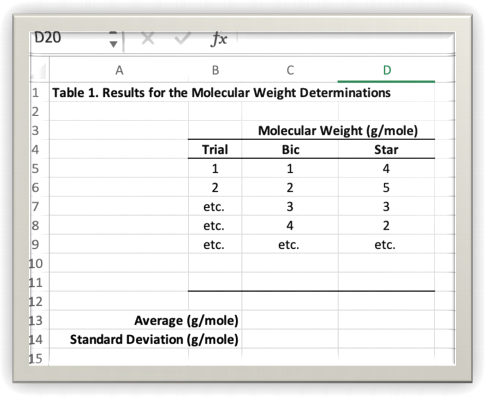
DID YOU SEE?
8. Prepare a data table and include the original data, and calculated columns for the initial sample
mass, final sample mass, mass loss, and the percent mass loss. Also calculate the average and
standard deviation of the percent loss. It is OK to use your judgement about whether you should exclude any points from the average. Prepare a table formatted in a similar manner to the table
shown above in figure 1. If you have difficulty deriving the correct formula for percent mass loss, or for that matter if you ever have a problem coming up with a “percent anything” formula, create your own similar problem that you can solve in your head, and then figure out what the formula is that got you to the answer. For example, here’s a problem that you can solve this problem in
your head, “If I used to weigh 100 kg and now I weigh 70 kg, then what is my percent mass loss?” Use your answer to this problem to figure out the correct formula required to calculate a percent mass loss. The formula that works for people is also good for baking soda.
Note: When tabulating data in Excel, the actual number of significant digits that the data was collected with should be shown. When tabulating calculations, the number of significant digits
displayed should be consistent down the column and can either be the correct number based on the rules for mathematical operations for significant figures (recommended), or more simply just display 3 sigfigs, but it is your choice. For final answers, only the correct number of sigfig should be shown. Please see the two examples below for determining significant figures for standard deviation.
![]()

9. How many significant figures will a standard deviation usually have?
10. Upon heating, if the baking soda reacted to give solid sodium carbonate along with water and carbon dioxide gas that left the reaction container, what would be the theoretical percent mass loss of a sample of baking soda upon heating to a constant weight?
11. When a scientist uses the term “error” it has a very specific meaning. Error is a measured value minus the true value.
Error = measured value – true value
This is a very simple concept, but since the word has a different meaning in everyday English, it causes students to struggle terribly. Calculate the error in the class average for heating baking
soda assuming the reaction described in the question above occurs to give you a “true” value. Calculate the percent error.
DID YOU SEE?
12. Using the Wikipedia value for the solubility of potassium alum in water at 20.5° C* that 14.00 g of alum will dissolve in exactly 100 mL of solution.
a. How much alum would you weigh out to prepare the hot solution as instructed in the lab manual?
b. Calculate how many grams of alum should crystallize from the hot solution as it cools to room temperature over the week (based on the amount from part a above).
c. What is the molarity of a saturated solution of alum at 20.5° C? You can assume that the saturated solution is close to 14% (w/v).
d. What is the concentration of aluminum in the saturated solution in the units of percent (m/v)?
2023-09-18