SSG Worksheet 1 – Fall 2023
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
SSG Worksheet 1 - Fall 2023
Instructions: Answer the following questions. Upload a copy of your work to Gradescope by Tuesday 9/12
at 6:25pm. As stated in our syllabus, late assignments will not be accepted as the answers will be
discussed at the SSG session. This worksheet will be graded based on overall completion. Only one of the following questions will be graded based on accuracy. An answer key will be available on LATTE after the due date.
1. Draw Lewis structures of the following molecules. Be sure to show the lone pairs and show all non-zero formal charges.
a. C2 H5OH
b. C2 H7 N
c. CH2Cl2
d. CO2
e. LiOH
f. (CH3CH2OH2)+
g. (CH2CN)-
h. NaNHCH3
2. Which of the compounds in Question 1 contain ionic bonds? Which compounds contain only covalent bonds?
3. Plenty of fruits have a sweet-smelling fragrance which comes from various esters. Answer the following questions regarding the Lewis structure of isopentyl acetate, which gives the sweet smell of banana!
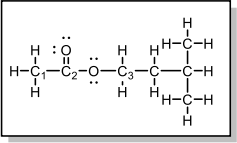
a. What is the hybridization of C1?
b. What is the molecular geometry of C1??
c. What is the hybridization of C3?
d. What is the hybridization of the O bonded to C3?
e. The sigma bond in C2=O is formed by the overlap of a(n) orbital from C and a(n) orbital from O.
f. The pi bond in C2=O is formed by the overlap of a(n) orbital from C and a(n) orbital from O.
g. Identify if the bond between O and C3 is sigma (σ) or pi (n).
4. Calculate the formal charge on each atom (N, C, O) in cyanate ion:
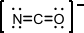
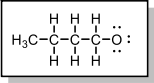
5. Assign the formal charge to the butoxide oxygen.
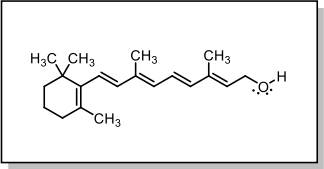
6. Vitamin A is an important part of the visual cycle that ultimately enables you to see. On the line structure below, circle all atoms with tetrahedral electron geometry.
7. A molecule's polarity can be determined by taking into account the polar bonds it contains, along with its molecular geometry. List the following molecules as polar or non-polar.
a. CO2
b. CH2Cl2
c. CCl4
d. C2 H2
e. CH2O
f. NH3
8. Which of the following statements is the best explanation for the shapes of atomic orbitals?
a. The shape of an orbital describes a surface that encompasses about 90% of the probability of finding an electron around a nucleus.
b. Orbital shapes have no physical meaning, but we draw different shapes to distinguish orbitals from each other.
c. Orbital shapes describe the path traveled by an electron as it moves around the nucleus.
d. The shape of an orbital encompasses all the possible locations of an electron around a nucleus.
e. None of the other statements correctly explains why orbitals have the shapes they do.
9. Which of the following orbitals is the best representation of asp3 hybrid orbital?

10. Convert the following molecular formulas to a (i) Lewis structure, (ii) structure with the correct geometry and orbitals involved in σ and π bonding. Be sure to show the lone pairs and show all non-zero formal charges.
Example: C2 H4
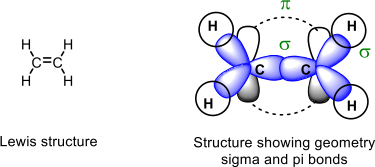
a) C3 H6
b) CH3CHO
11. Draw the molecular orbital for F2 showing the combinations of the 1s, 2s, and 2patomic orbitals to form molecular orbitals. Label each energy level with σ, σ*, π, π* as appropriate.
2023-09-13