Quantum Mechanics Study of Sodium Ion Binding
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
LI Year 2 Chemistry Options: Introduction to Computational Chemistry
Topic 15: Quantum Mechanics Study of Sodium Ion Binding
1. Using WebMO (https://www.webmo.net/demo/), import the benzene-Na+ and benzene geometries that are already optimized and can be downloaded from this Topic’s in-lecture page on Canvas. Calculate the binding energy (in kcal/mol) between benzene and Na+ ion [Note: for calculating the energy of Na+ , perform a third calculation for a sodium atom with its Charge set to 1]. Use HF/6-31G(d) for all calculations – have your initials in job names.
|
(1) SCF energy of Na+ = - 161.6593 Hartree (2) SCF energy of benzene = -230.7028 Hartree (3) SCF energy of benzene– Na+ = -392.4052 Hartree SCF binding energy = (3) - (1) - (2) = -0.0431 Hartree = -27.0456 kcal/mol (1 Hartree = 627.5095 kcal/mol) |
2. Following the same procedure, calculate binding energies for the cyclohexane-Na+ complex. Compare the three aromatics’ binding strengths with Na+ . Use HF/6-31G(d) for all calculations – have your initials in job names.
|
(1) SCF energy of Na+ = - 161.6593 Hartree (2) SCF energy of cyclohexane = -234.2080 Hartree (3) SCF energy of cyclohexane– Na+ = -395.8819 Hartree SCF binding energy = (3) - (1) - (2) = -0.0146 Hartree = -9.1616 kcal/mol (1 Hartree = 627.5095 kcal/mol) |
3. Calculate electrostatic potentials for benzene and cyclohexane, using HF/6-31G(d). Use thus- obtained electrostatic potentials to discuss the location of Na+ with respect to the organic molecule and the calculated binding strength.
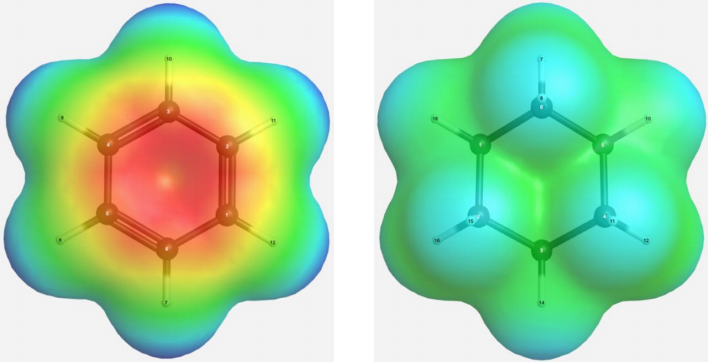
|
|
|
|
The more negative (redder) the electrostatic potential, the higher the electron density; the more positive (bluer) the electrostatic potential, the lower the electron density. Na+ is positively charged and, therefore, binds strongly to benzene through energetically favourable π-cloud–cation electrostatic interactions. |
|
2023-08-14