EMS525U Functional Materials CW1 Title
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
EMS525U Functional Materials
Experimental Coursework 1. Tuesdays, 14th,(group A) 21st (group C), 28th March (group C) and 4th (reserved day for technical failure in previous days and students with approved EC). Students will be split in 2 group of 3 students each. Each group carries out their own set of experiments.
Time 9.45 am – 2.45pm. Intro (10 min) starts strict at 9.45am
Synthesis and spectroscopic characterisation of carbon nanostructures.
Carbon nanoparticles will be synthesized by hydrothermal procedure with autoclaving three solution with sources of carbon: glucose, dextran and dextran sulfate solution at 180°C for 5 hours. Glucose, Dextran and dextran sulfate are used as source of carbon components and after hydrothermal procedure is converted to fluorescent carbon nanostructures. Fluorescent properties of carbon nanoparticles are to be examined by fluorimetry.
Description of procedure.
1. Prepare solutions of 10 mg/ml of each of the component (glucose, dextran and dextran in ultrapure water in plastic tube.
2. Place the tube in a metal vessel for autoclaving for 5 hours (in room B20 – will be let by TA). At the end you will make photos of the solutions. In order to start the experiments you will be given 3 prepared solution of these components.
3. The transparent solution should become slightly dark indicating formation of carbon nanoparticles. You will see at the end and it should be looking like the solution given to you
4. Make UV-Vis spectroscopy measurements on all three samples in room G29. Get set of absorption measurements (like Figure a)
5. Move to 2nd floor to fluorimeter for fluorescent measurements.
6. Conduct fluorescent measurements with different excitation wavelength. Experiments are to be done in cuvette. The results of spectra should look like on figure b:
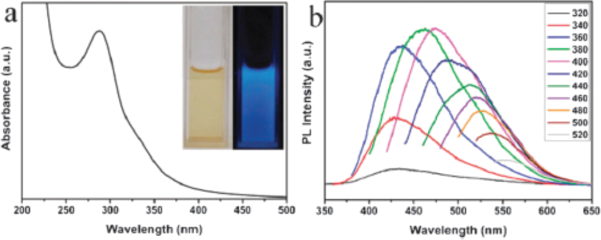
7. Collect these data together with the photographs of the samples.
8. It is expected that all group members have the same set of experimental raw data to write individual reports
At the CW submission point, each student should submit an individual report. The report (up to 3 pages including experimental data) on Synthesis and Characterisation of Carbon Nanoparticles should include
Title
Abstract (100 words) summary of your work
Introduction. Short review of carbon nanoparticles, its synthesis and application, cited literature should be included (up to 10 references)
Experimental part. Describe materials and methods you used, chemicals and procedure, UV-Vis spectrometer and Fluorimeter.
Results and Discussion. Describe and analyse your results. Discuss why you get the data shown on your graphs; how are carbon nanoparticles different from quantum dots; what does the difference in fluorescence spectra taken at different excitation wavelengths mean? Comparison with data in literature (cited).
Conclusion. (up to 200 words) What did I learn in this CW?
References (not less than 10)
Marking scheme:
Proper attendance and involvement in experiments, Preparation, work in lab, commitment to conduct experiments, effectiveness, working with other group members. (assessed by TA; absence from the labs will result in 0) 5 marks
Abstract. Adequate summary covering whole report 2 marks
Introduction. Given background of carbon nanoparticles, its synthesis, properties and application as outlined from cited papers 5 marks
Experimental details. All materials, procedures and methods are described in sufficient detail. 4 marks
Results and Discussion. Illustrative and correct presentation of graphs and photos, 5 marks
Proper description of data and comparison with literature data 6 marks
Conclusion. Adequate description of what student learnt in CW, what skills gained and what data produced 3 marks
References. 3 marks (10 relevant references means full mark for that)
All together CW worth 33 marks
2023-08-01
Synthesis and spectroscopic characterisation of carbon nanostructures.