ENGR 145 Homework unit 3
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
ENGR 145
Homework unit 3
1. Sketch the ψσ and ψσ* MOs between the following pairs of atomic orbitals. Also sketch the electron probability distribution (ψ2) for each MO, along the line going through the two nuclei.
a. an s orbital on one atom and an s orbital on another atom
b. a p orbital on one atom and a p orbital on another atom
c. an s orbital on one atom and a p orbital on another atom
2. For Cl2, draw the MO diagram, including the occupancy of electrons.
a. Identify bonding and anti-bonding orbitals.
b. Identify the HOMO and the LUMO.
c. What is the bond order?
3. For carbon monoxide (CO), draw the MO diagram, including the occupancy of electrons. Be sure to depict the correct relative energies of the C and the O atomic orbitals.
a. Identify bonding and anti-bonding orbitals.
b. Are the bonding orbitals more like the C atomic orbitals or the O atomic orbitals?
c. Identify the HOMO and the LUMO.
d. What is the bond order?
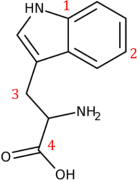
4. The tryptophan molecule is shown below.
a. How many carbon atoms are in the molecule? How many hydrogen atoms?
b. For each of the atoms marked 1-4, identify the hybridization state and how many sigma bonds the atom forms.
c. Identify any localized pi bonding.
d. Identify any delocalized pi bonding (disregard any possible participation of non-C atoms).
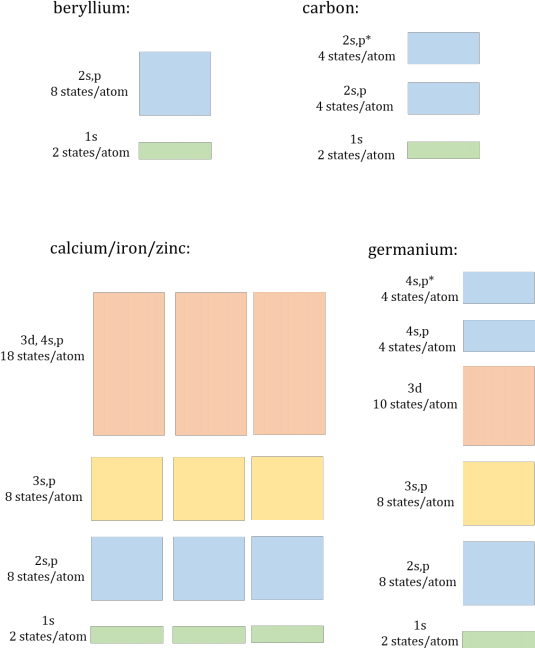
5. Draw the occupancy of electrons in the energy bands diagrams for beryllium, carbon, calcium, iron, zinc and germanium. Identify each as a conductor or insulator/semiconductor.
6. Callister 12.23. In addition, compare GaP vs ZnS
2023-07-13