ENGR 145 Exam 1
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
ENGR 145
Exam 1
1. Consider an electron in the potential below. Between x=0.4 nm and 0.7 nm, the potential has the value Vb= 0.2*Npersonal
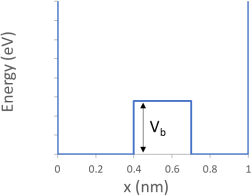
Please write your value of Vb here:
a. What is the energy of the quantum state with one node? Your answer must be correct to within ±0.01 eV to get full credit.
b. Paste a screen shot of the wavefunction (or you can upload the screenshot to Canvas separately if you’re writing on a printed copy of the exam)
c. Is this wavefunction most like an s, p, d or f orbital, and why?
d. Does tunneling occur in this situation? Briefly explain how you got your answer.
e. On the plot below, sketchEx the probability distribution for this electron.
f. The energy unit in this problem is eV. This is not an SI unit.
i. What is the advantage of NOT using SI units in this problem?
ii. What is the key advantage of SI units?
g. Write out the 1-dimensional Schrodinger equation in terms of the potential energy V(x), and explain how it can be considered a quantum mechanical version of the conservation of energy equation.
2. Solve the problem below for an atom with atomic number N=20+Npersonal
Please write your value of N here:
a. Draw the energy level diagram and include arrows in the diagram indicating the distribution of electrons (and their spin states) in the orbitals of this atom. The energy level diagram must be drawn such that energy increases from the bottom to the top of the page, and energy doesn’t change going from left to right on the page.
b. What is the electron configuration for this atom?
c. How many valence electrons are there, and what orbitals are they in?
d. How is the order in which the 3d, 3p and 3s orbitals are filled with electrons determined by the three-dimensional distributions of the orbitals?
e. The 1s orbital has the lowest energy. Why is it that all electrons do not go in the 1s orbital? Your answer should be a description of the physical basis… NOT just saying that only two electrons can go in an orbital or saying “Pauli exclusion principle” (which is just the name of the effect).
f. When things like s2, p6 and d10 are written… Why does s have a 2; p have a 6; and d have a 10? Give your answer in terms of the quantum numbers n, l and/or m.
g. Which has the lower (more negative) energy: the 1s orbital for your atom (atomic number N=20+Npersonal) or the 1s orbital of Ca? Give the physical explanation for your answer.
h. In 20 words or less, explain how this problem is related to the Schrodinger equation
2023-07-12