Enzymology Laboratory
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
Introduction and Laboratory Report
The purpose of this Virtual Laboratory is to test your ability to carry out an experiment, interpret the results and write a laboratory report. You are required to plan and carry out a virtual experiment. You should record your results and then write a lab report for your tutor, detailing your methods, results, discussions and conclusions. You must describe any safety precautions that would be taken in a physical lab and list all steps taken to ensure a fair test. Refer to your lab manual for experimental instructions.
Your lab report should contain the following sections:
Introduction. You should give a brief overview of the main theories behind the experiment. What is the background to this experiment?
Aim. Clearly state the aim of the experiment – what are you trying to determine?
Materials and methods. You should explain how the experiment was carried out. You should give enough detail so that another person can repeat the experiment in the same way you did.
Results. You must include all your results. Results tables should be clearly labelled. All measurements must include units. If graphs are required, they should be an appropriate size and clearly labelled.
Discussion. You need to explain what your results mean. You should link your results to relevant theories and explain how they fit together. You must use in-text citations where appropriate. Any unexpected results should be explained.
Conclusion. State what has been learned from this experiment. Have you met the aim of the experiment?
Errors. What may have affected your results? This section should include errors such as systematic, parallax and human errors – ones that cannot be avoided.
References. You should list all sources used.
Theory and/or task resources required for the assessment:
You should refer to the appropriate chapters in the key texts as well as the lecture materials for the topic relevant to your experiment. You should also find relevant primary sources online using the online resources provided by your host university.
Referencing style:
You should refer to a minimum of 5 relevant sources for your report. Minimum 2 sources should be printed texts.
You must include a Harvard style reference list at the end of your report.
Expected word count:
Your overall word count should be 1500-2000 words, excluding references.
In each section you should include around the following number of words:
· Introduction & Aim 300-500 words
· Materials and methods 300 words
· Results 200 words
· Discussion 500-600 words
· Conclusion & Errors 200 words
Submission Requirements:
You must include the following paragraph on your title page:
I confirm that this assignment is my own work.
Where I have referred to academic sources, I have provided in-text citations and included the sources in the final reference list.
You must type your assessment in Arial font 11, with single spacing.
Save your file as a MS Word file and name it using this format:
YOUR STUDENT NUMBER – MODULE CODE – LabReportFile(n)-21.05.20,
eg: TT225566- FC345-LabReportFile(1)-21.05.20
You must submit the assessment electronically via the VLE module page. Please ensure you submit it via Turnitin.
Assessments submitted after the submission deadline may incur penalties or may not be accepted.
Enzymology Laboratory
Aim: In this virtual lab, we will perform a series of experiments to assess the optimal conditions for catalytic activity of an enzyme.
Introduction
In biological systems, enzymes act as catalysts and play a critical role in accelerating reactions, anywhere from 103 to 1017 times faster than the reaction would normally proceed. Enzymes are high-molecular weight proteins that act on a substrate, or reactant molecule, to form one or more products.
Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. For example, the enzyme acetylcholinesterase catalyses the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Many enzyme–substrate reactions follow a simple mechanism that consists of the initial formation of an enzyme–substrate complex, ES, which subsequently decomposes to form product, releasing the enzyme to react again.
Acetylcholinesterase (AChE) may be one of the fastest enzymes. It hydrolyses acetylcholine to choline and an acetate group. One of the earliest values of the turnover number was 3×107 (molecules of acetylcholine) per minute per molecule of enzyme. A more recent value at 25°C, pH = 7.0, acetylcholine concentration of 2.5×10−3M, was found to be 7.4×105min−1 (J Biol Chem. 236 (8): 2292–5.).
There may be some 30 active centres per molecule. AChE is a serine hydrolase that reacts with acetylcholine at close to the diffusion-controlled rate. A diffusion-controlled reaction occurs so quickly that the reaction rate is the rate of transport of the reactants through the solution; as quickly as the reactants encounter each other, they react.
This is described within the following multi-step mechanism:
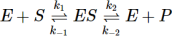
The formation of the enzyme substrate complex can be described as a relationship between the concentration of enzyme, substrate and the constant of dissociation and association. The relationship between the rate constants of dissociation and association of the enzyme is called Michaelis constant (Km).
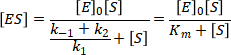
For high substrate concentrations, where [S] >> Km, the equation simplifies to:
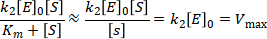
where Vmax is the maximum rate for the catalysed reaction. Under these conditions the reaction is zero-order in substrate, and we can use Vmax to calculate the enzyme’s concentration.
The Michaelis constant Km is the substrate concentration at which the reaction rate is at half-maximum and is an inverse measure of the substrate's affinity for the enzyme—as a small Km indicates high affinity, meaning that the rate will approach Vmax more quickly. The value of Km is dependent on both the enzyme and the substrate, as well as conditions such as temperature and pH.
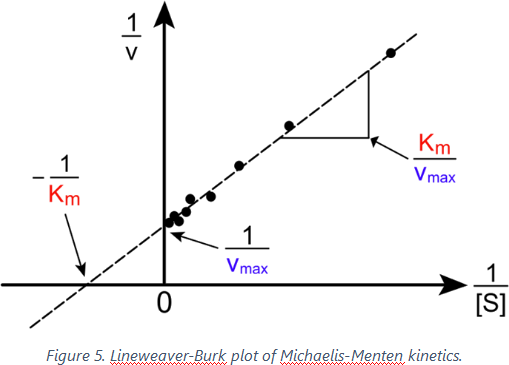
A commonly used plot in examining enzyme kinetics is the Lineweaver-Burk plot, in with the inverse of the reaction rate, 1/v, is plotted against the inverse of the substrate concentration, 1/[S].
The Lineweaver–Burk plot (or double reciprocal plot) is a graphical representation of the Lineweaver–Burk equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934. The Lineweaver-Burk plot results in a straight line with the slope equal to KM/k2[E]0 and y -intercept equal to 1/k2[E]0 which is 1/Vmax (Figure 5).
Questions to discuss:
1. Why does pH affect the activity of an enzyme?
2. Why does temperature affect the activity of an enzyme?
3. Why does concentration affect the catalytic activity of an enzyme?
4. Describe the enzyme that you have chosen. Is it thermoresistant? Is it pH sensitive? Is it pH tolerant? Is it thermosensitive?
5. Why are the Km and Vmax useful values to compare enzymatic activity?
Justify your answers to the following questions:
6. Which of the following would interfere most with the ability of an enzyme to catalyse a reaction?
a. Reduced concentration of substrate available
b. Reduced concentration of product available
c. Increased concentration of substrate available
d. A change in the pH
7. Feedback mechanisms regulate the rate of enzyme activity, effectively “turning off” an enzyme in a reversible way until more product is needed. Which of the following would be most effective as a feedback mechanism?
a. Reduced concentration of product
b. Increased concentration of substrate
c. A change in pH
d. Temporary binding of a non-substrate molecule in the active site
2023-07-11