CHEM 150 CONCEPTS IN CHEMISTRY FIRST SEMESTER, 2017
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
CHEM 150
FIRST SEMESTER, 2017
Chemistry
CONCEPTS IN CHEMISTRY
QUESTION 1: (14 marks TOTAL)
(a) (2 marks) Classify each of the following as elements (E), compounds (C) or Mixtures (M).
Write the letter X if it is none of these. Each part is worth ½ marks.
(i) Concrete ___M____
(iii) Bismuth ___E____
![]() (ii) Carbon dioxide C
(ii) Carbon dioxide C
(iv) Energy ___X_____
(b) (2 marks) Classify each of the following as a physical (P) or a chemical (C) property. Each
part is worth ½ marks.
(i) Lead becomes a liquid when heated to 601oC _____P_____ (ii) Bleach turns hair yellow _____C_____
(iii) Milk turns sour on standing at room temperature _____C_____
(iv) Ice floats on top of liquid water _____P_____
(c) (1½ marks) Name the following allotropes of carbon on the line provided.
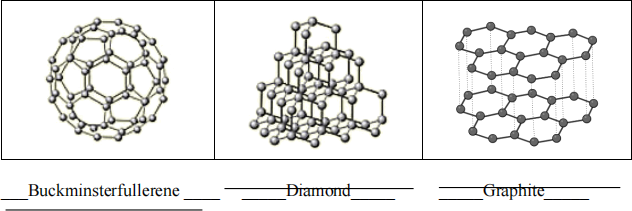
(d) (1 mark) What is the density of a salt solution if 75.0 mL of the solution has a mass of 32.0g?
Density = mass/volume = 32.0 g/75.0 mL = 0.427 g mL- 1
(e) (3 marks) For each of parts (i) – (vi), write the answer on the line provided. Each part is
worth ½ mark.
(i) Which subatomic particle has the smallest mass? Electron
(ii) Which part of an atom has the highest density? Nucleus
(iii) Which subatomic particle(s) are responsible for the volume of an atom Electrons
(iv) Which subatomic particles are responsible for the mass of an atom Protons, Neutrons
(v) Name the process in which a gas (vapours) is converted to a liquid condensation
(vi) Name the process in which a solid is converted to vapour phase sublimation
QUESTION 1 (continued):
(f) (1½ marks) Complete the following sentence about an atom of 238U.
This atom has __92_____ protons, __ 146_____ neutrons and __92____ electrons.
(g) (3 marks) For parts (1) to (6), write the number of the best answer on the line provided.
Each part is worth ½ marks.
1. Which of the following statements is correct?
(i) Carbon is a mixture and air is a compound (ii) Table salt is a mixture and carbon is a compound (iii) Air is a mixture and carbon is a compound
(iv) Air is a mixture and water is a compound ___(iv)____
2. The formula for copper sulphate is CuSO4 . What is the ratio of Cu : S : O atoms in the formula?
(i) 1 : 4 : 4 (ii) 1 : 1 : 4 (iii) 4 : 3 : 4
(iv) 4 : 4 : 4 ___(ii)____
3. Rutherford's experiment with alpha particle scattering by gold foil established that
(i) protons are not evenly distributed throughout an atom (ii) electrons have a negative charge
(iii) atoms are made of protons, neutrons, and electrons
(iv) protons are 1840 times heavier than electrons ___(i)____
4. Which of the following are isotopes?
(i) 12C and 12CO (ii) 14C and 13C (iii) 14N and 14N2
(iv) 14N and 14N3- ____(ii)____
5. A technique in which two liquids, in a mixture, are separated by heating process is known as
(i) condensation (ii) filtration (iii) evaporation
(iv) distillation ____(iv)____
6. How many protons (p) and electrons (e) are present in one Br– ion?
(i) 35 p, 35 e (ii) 80 p, 81 e (iii) 35 p, 34 e
(iv) 35 p, 36 e ____(iv)_____
QUESTION 2: (16 marks TOTAL)
(a) (1 mark) Complete the following sentences by writing the appropriate word(s) on the lines.
Each part is worth ½ marks.
(i) The volume of a sample of a gas is inversely proportional to the pressure of the gas at constant temperature.
(ii) The volume of a sample of a gas is directly proportional to its temperature
at the gas at constant pressure.
(b) (3 marks) Write the electron configuration using s, p, d notation for the following:
(i) As 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3 (ii) As3- 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 (iii) Explain why the radius of a As3- ion is bigger than the radius of a As atom.
As3- has 33+3= 36 electrons whereas its nucleus contains 33 protons only. As3- radius is larger because of electron-electron repulsion and weaker pull by nucleus
(c) Write the name for each of the following compounds on the line to the right:
(i) (½ mark) NH4NO3 Ammonium nitrate
(ii) (½ mark) PCl5 Phosphorus pentachloride
Write the formula of the following compounds on the line to the right.
(i) (½ mark) Dinitrogen monoxide __________N2O____
(ii) (½ mark) Aluminum sulfide _____________Al2 S3_
(d) (1 mark) Explain briefly why members of group 17 (halogens) of the periodic table exhibit similar chemical properties
Because the halogen elements have seven valence electrons, they only require one additional electron to form a full octet. This characteristic makes them more reactive than other non-metal groups. They all react very vigorously with alkali metals - salt producer.
(e) (2 marks) Balance the following precipitation (reaction) equations by placing coefficients
on the lines provided. Circle the products that forms precipitates.
(i) ___ 1__CaCl2(aq) + ___2__AgNO3(aq) ![]() __ 1___ Ca(NO3)2 + __2__AgCl
__ 1___ Ca(NO3)2 + __2__AgCl
(ii) ___3__KOH(aq) + ___ 1__ FeCl3(aq) ![]()
![]() 3
3![]() KCl + __ 1___Fe(OH)3
KCl + __ 1___Fe(OH)3
(f) (1 mark) Name the type of intermolecular force that is responsible for the attraction
between an ‘ion’ and a ‘polar molecule’?
Ion-dipole
QUESTION 2 (continued):
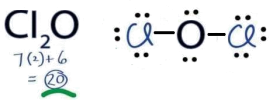
(g) (2 marks) Draw the Lewis structure for Cl2O molecule in the space below.
(h) (2 mark) Indicate whether copper is oxidised or reduced in the following reaction.
CuO(s) + H2(g) ![]() Cu(s) + H2O(l) oxidized / reduced (circle one)
Cu(s) + H2O(l) oxidized / reduced (circle one)
Justify your answer
|
Reduced -- Cu2+ has gined 2 electrons from hydrogen molecule and changed to Cuo (metal) |
(i) (1 mark) Define the term ‘vapour pressure’ .
|
Vapour pressure of a liquid is the pressure of gas (vapour) above liquid in a closed container at a given temperature |
(j) (1 mark) A polar covalent bond would form in one of the following pairs of atoms? (Circle
the correct choice).
Cl — Cl Si — Si Ca — Cl Cr — Br P — Cl
Justify your choice.
|
A polar covalent bond forms when atoms with different electronegativity values combined by sharing of electrons |
QUESTION 3 (20 marks)
(a) Compound M is shown right,![]()
(i) (1 mark) Compound M is classified as:
saturated / unsaturated (Circle one.)
Justify your choice.
Doube bonds present.
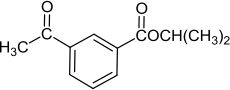
Compound M
(ii) (1 marks) The three oxygen containing functional groups in compound M are:
_____k_e![]()
![]() _n_e_____ and ___
_n_e_____ and ___![]() _s
_s![]()
![]()
![]() _____
_____
(iii) (1½ marks) Complete the molecular formula for compound M. C_1_2__H1__4__O_3___
(iv) (1½ marks) Draw Compound M as a line structure.
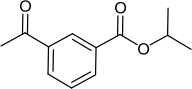
![]()
(b) (6 marks) Consider the structures below:
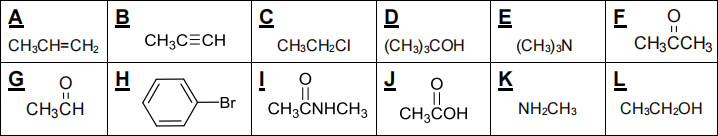
Give, in the space provided, the letter of one compound which:
(i) is an aldehyde __ G ____ (ii) is an amide I _____ (iii) is an alkyne ___B______ (iv) is a tertiary alcohol ___D______
(v) is aromatic ___H______ (vi) is a hydrocarbon A o__r_ B
![]() (vii) can be oxidised by PCC L
(vii) can be oxidised by PCC L
L
(viii) can be formed from compound C via a substitution reaction? _________
(ix) Which two compounds from the grid can react to give compound I? _ E _ and _J____
(x) Which of the following compounds from the D / E / G / K grid can form hydrogen bonds? (circle as appropriate)
(c) The following questions refer to the reaction scheme below.
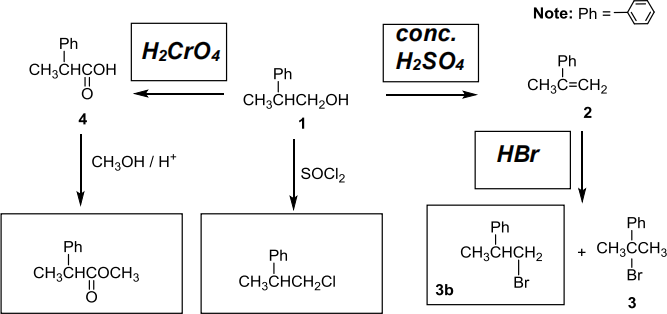
(i) (4 marks) Complete the reaction scheme by filling in the missing reagents and products.
(ii) (1 mark) The reaction that produces 3 produces a second brominated product (3b).
Draw the structure of this product in the box provided.
(iii) (½ mark) The reaction going from compound 2 to compound 3 is a
substitution / elimination / addition
(circle one)
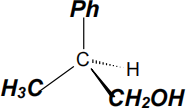
(iv) (1 mark) Complete, to the right, the three dimensional representation for one enantiomer of compound 1.
(b) The following questions refer to the reaction scheme below:
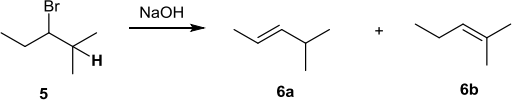
![]()
(i) (½ mark) On compound 5 (above) draw in the hydrogen that is eliminated to form compound 6b.
(ii) (½ mark) On compound 5 (above) indicate the stereocentre with an asterisk (*).
(iii) (½ mark) Which compound(s) in the scheme above can exist as E/Z isomers?
6a only
(iv) (½ mark) Compounds 6a and 6b are c__o__ns___titu____tio___n__al___isomers of each other.
(v) (½ marks) Give the systematic name for compound 6b 2__-_me____t_![]()
![]() _l_
_l_![]()
![]() _nt-___2-___ene
_nt-___2-___ene
QUESTION 4 (14 marks in Total)
(a) In 1950s pentaborane-9, B5H9 was considered as a potential rocket fuel. It is a highly
reactive liquid that will burst into flame or even explode when exposed to oxygen. The reaction is
2B5H9 (l) + 12O2(g) → 5B2O3 (s) + 9H2O(g)
(i) (1 mark) Show that the molar mass of pentaborane-9, B5H9 is 63.122 g mol- 1 .
= 5 × 10.81 + 9 × 1.008 = 63.122 g mol-1
(ii) (1 mark) Calculate the amount in moles of pentaborane -9, B5H9 in 1.252 kg.
n = 1.252 × 103 g / 63.122 g mol-1 = 19.8346 mol = 19.835 mol
(iii) (2 marks) Calculate the mass in kg of H2O produced in this reaction.
n[H2 O] [B5H9 ] n[H2 O] [19.835 mol]
= =
9 2 9 2
![]() 19.835 mol
19.835 mol
2
m(H2O ) = 89.2575 mol × 18.016 g mol-1 = 1608.063 g = 1.608kg
(b) Urea , (NH2)2CO is produced by reacting ammonia with carbon dioxide as shown by the
following equation.
2NH3(g) + CO2 (g) → (NH2)2CO(aq) + H2O(l)
(i) (2 marks) In one process 637.2 g of NH3 are treated with 1142 g of CO2 . Which of the two reactants is the limiting reagent? M(NH3) =17.03 g mol- 1, M(CO2) = 44.01 g mol- 1 . Show your working.
n(NH3) = 637.2 g / 17.03 g mol-1 = 37.416 mol
n(CO2) = 1142 g / 44.01 g mol-1 = 25.949 mol
To react with 37.416 mol of NH3 there should be 37.416 / 2 mol = 18.708 mol of CO2 There is 25.949 mol of CO2. The limiting reactant is NH3
(ii) (2 marks) Calculate the mass of (NH2)2CO produced in this reaction. M((NH2)2CO) = 60.06 g mol- 1
n(NH2)2CO = 37.416 mol / 2 = 18.708 mol
m(NH2)2CO = 18.708 mol × 60.06 g mol-1 = 1123.6 g = 1124 g
(iii) (2 marks) Calculate the mass of excess reagent left at the end of the reaction.
Moles of CO2 used = 18.708
Moles of CO2 left = 25.949 – 18.708 = 7.241 mol Mass of CO2 left = 7.241 mol × 44.01 g mol-1 = 318.7 g
QUESTION 4 (continued):
(c) Acidity of Coca Cola is due to the presence of phosphoric acid, H3PO4 . This reacts with sodium hydroxide as shown below.
H3PO4(aq) + 3NaOH(aq) —![]() Na3PO4(aq) + 3H2O(l)
Na3PO4(aq) + 3H2O(l)
Content of a 330 mL Coca Cola can is titrated with 0.338 mol L- 1 sodium hydroxide. It required 23.25 mL of NaOH for the complete neutralization.
(i) (1 mark) Calculate the amount in moles of NaOH used in the titration.
n(NaOH) = 0.338 mol L-1 × 0.02325 L = 7.8585 × 10-3 mol
(ii) (2 marks) Calculate the amount in moles of H3PO4 in a can of Coca Cola.
![]() n(H3PO4 ) n(NaOH) 7.8585
n(H3PO4 ) n(NaOH) 7.8585 ![]() 10-3 mol -3
10-3 mol -3
1 3 3
(iii) (1 mark) Calculate the concentration of H3PO4 in a can of Coca Cola.
C(H3PO4 ) = ![]() =
= ![]() 7.94
7.94 ![]() 10-3 mol L-1
10-3 mol L-1
QUESTION 5 (13 marks in Total)
(a) Diborane, B2H6 was once considered as a possible rocket fuel for the space program.
B2H6 is synthesised as given by the following equation.
2B(s) + 3H2(g) → B2H6(g)
(i) (3 marks) Calculate the enthalpy change for the above reaction using the information given below.
4B(s) + 3O2(g) → 2B2O3(s)
H2(g) + ½ O2(g) → H2O(g)
B2H6(g) + 3O2(g) → B2O3(s) + 3H2O(g)
![]() H° = -2546 kJ
H° = -2546 kJ
![]() H° = -286 kJ
H° = -286 kJ
![]() H° = -2035 kJ
H° = -2035 kJ
|
2B(s) + 3/2 O2(g) → B2O3(s)
3H2(g) + 3/2 O2(g) → 3H2O(g)
B2O3(s) + 3H2O(g) → B2H6(g) + 3O2(g)
|
(ii) (1 mark) If the B2H6 synthesis is carried out in a calorimeter would the temperature
of the calorimeter stays the same, increase or decrease?
Justify your answer
|
Temperature will increase as the reaction is exothermic |
2023-06-25