CHEM0034
Hello, dear friend, you can consult us at any time if you have any questions, add WeChat: daixieit
CHEM0034
1. Answer ALL parts.
(a) For the two reactions below give the mechanism for the formation of the product shown and explain any selectivity.
(i)
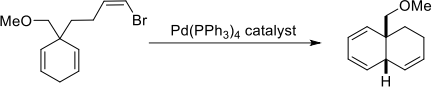
(ii)
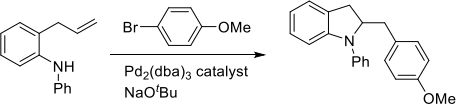
(b) Give the product of the following transformation. The first step of the catalytic cycle is an oxidative addition reaction. Explain all mechanisms and justify any selectivity.

(c) Give the product arising from an initial Wacker-type addition on the substrate below followed by subsequent steps. Explain all mechanisms and justify any selectivity.
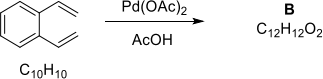
2. Answer ALL parts.
(a) Reaction of epoxide A with sodium azide followed by work-up gives B, which after treatment with LiAlH4 gives C.
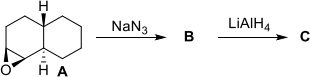
(i) Draw a 3D half-chair representation of epoxide A.
(ii) Provide a structure for B, give a mechanism for its formation and explain any regioselectivity or stereoselectivity in the reaction.
(iii) Draw a 3D conformational diagram of C.
(iv) Suggest a synthesis of a diastereoisomer of C from epoxide A.
(b) (i) Draw three-dimensional structures showing the stereochemistry of compounds D to I in the following reaction sequence. Explain any stereoselective or regioselective reactions. Mechanisms are NOT required.
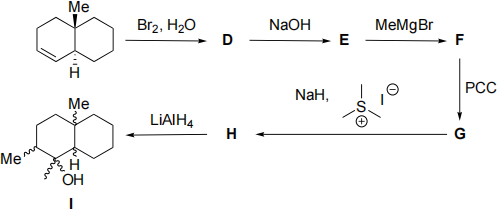
(ii) What product would be formed if compound H were treated with LiAlD4 instead of LiAlH4?
(c) Provide a synthesis of compound J from D-glucose drawing three-dimensional structures of all intermediate compounds on the synthetic route and explaining any stereoselective or regioselective reactions. More than one step will be required. Mechanisms are NOT required.

3. Answer ALL parts.
(a) (i) Predict the major product formed from the following carbonyl addition reaction clearly showing the transition state and explaining the expected stereoselectivity.
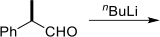
(ii) Predict and explain the major enolate formed from the following reaction.
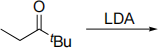
(b) Label the sigmatropic rearrangement below as [i,j] where i and j are integers. Draw frontier molecular orbitals to explain why this sigmatropic rearrangement can occur photochemically, but not thermally.
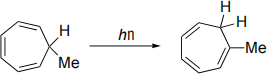
(c) The following thermal reaction takes place by two consecutive electrocyclic ring closures. Predict the structure of intermediate A and the stereochemistry in the final product by drawing the mechanisms and the frontier molecular orbitals involved.
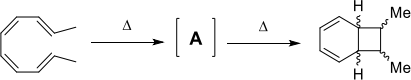
2023-05-08